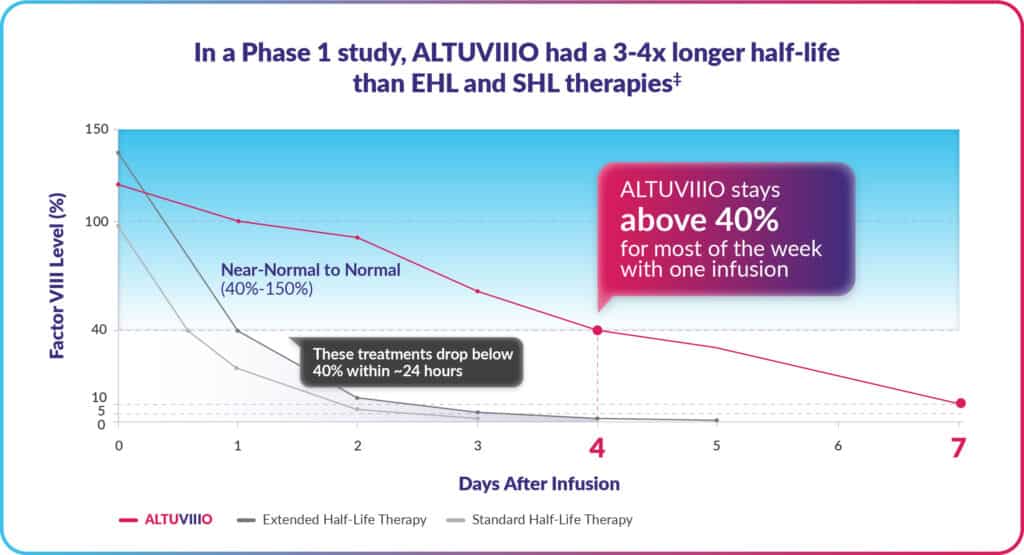
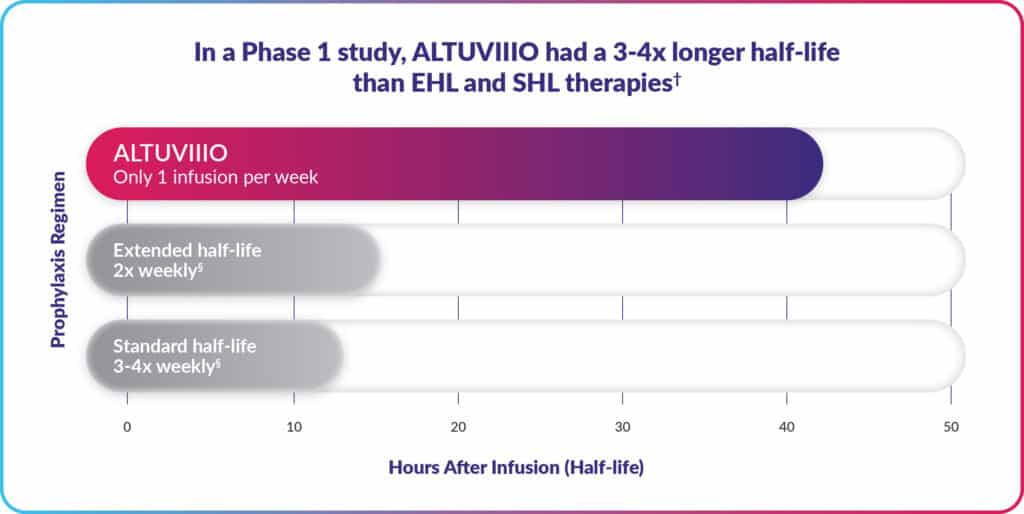
Women and hemophilia: evolving knowledge and raising awareness
I love sharing stories from our community, and have been a voice for hemophilia patients since 1990, when we published Raising a Child with Hemophilia. It included stories from 125 families, the first ever such publication. Let’s continue that tradition! In this blog, Sanofi introduces you to a woman with hemophilia, Kyrie, who shares her unique story.
Sponsored by Sanofi
While hemophilia is more common in men, women can also experience symptoms and receive a diagnosis. Because of a traditional misconception that women are only carriers of hemophilia, they have historically not been tested and treated for the condition.1 This is changing, thanks to emerging understanding and awareness of hemophilia in women.
Kyrie Smith lives with hemophilia and works as a Community Relations and Education (CoRe) manager at Sanofi. We sat down with her to hear about her hemophilia journey.
Please note that Kyrie’s story is hers alone. While there are common threads, everybody’s experiences with hemophilia will be different.
A legacy of advocacy
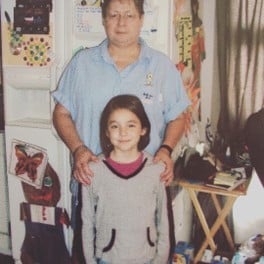
Kyrie’s family history with hemophilia goes back to her grandmother. According to Kyrie, her grandmother was one of the first women to receive access to on-demand factor treatment for bleeds as they occurred. Kyrie credit’s her grandmother’s self-advocacy with helping her get treatment at a time when it was uncommon for women to be diagnosed, let alone treated.
“The ability for my grandmother to be prescribed factor to control a bad leg bleed was quite novel at the time,“ Kyrie says. “While she is no longer with us anymore, I often find myself thinking about her strength and resilience.”
Hemophilia affects both women and men in Kyrie’s family. Since Kyrie was a carrier of hemophilia and could become symptomatic, her mother advocated for Kyrie’s factor activity levels to be checked during her brother’s annual appointments.
“I’m thankful for my mother’s persistence, as I was diagnosed so early in my childhood, when many women— including my mother—are often diagnosed later in life,” Kyrie says. “I am very proud to come from a long line of strong women who have advocated for the health of their family as well as themselves.”
Strength in sisterhood
Kyrie was first diagnosed as a symptomatic carrier of hemophilia and was prescribed a treatment to address bleeds and other symptoms as they occurred. A few years later, her diagnosis was updated to mild hemophilia.
“This change was due to the strong women and advocates in the hemophilia community that helped evolve the definition of symptomatic carrier to mild hemophilia, which is defined by factor activity levels,” Kyrie says. “I am honored to now join them in their work to increase visibility and voices of women with hemophilia.”
By working with her healthcare team, Kyrie has been able to manage her condition. That doesn’t mean, however, that the situation is without its challenges.
“From missing sports practices in high school to switching cardio workouts to avoid overworking my joints and managing pain, I’ve had to make some compromises along the way,” Kyrie says.
She’s grateful for the support and encouragement she’s received from the hemophilia community to help her get through difficult times. Kyrie started attending hemophilia camps at age 7, an experience she considers central to her continued sense of community.
“By having a mentor to look up to, or a peer to talk through an issue with, I’ve made lifelong friends from my time at hemophilia camps and other chapter or national events,” says Kyrie.
When to start asking questions
Awareness, testing, and diagnosis of hemophilia in women are increasing.1 It can be difficult, however, to know whether the bleeding you experience is normal or abnormal. When in doubt, it’s best to speak to your doctor to discuss what’s going on.
The common signs of hemophilia in women include:2
- Heavy menstrual periods, such as soaking through one or more pads or tampons every 2 hours or less3
- Low in iron or have anemia
- Frequent nosebleeds that last longer than 10 minutes
- Bleeding from cuts lasting longer than 5 minutes
- Easily bruised (raised and larger than a quarter, happening on a weekly basis)
- Joint bleeds (pain, swelling, unusual sensations, warmth, loss of motion)
- Family history of a bleeding disorder
- Heavy bleeding from surgery
If you or someone you care about is experiencing the signs and symptoms above, consider reaching out to a doctor to explain what’s happening and get more information.
Coming full-circle
Kyrie now works as a Sanofi CoRe, which gives her the opportunity to pay forward the information and connection she has experienced from within the hemophilia community.
“Because the hemophilia community has played such a large role in my life, I want to continue giving back, being a resource for the next generation,” Kyrie says. “As a CoRe, I am able to connect with various members of the community no matter where they might be in their hemophilia journey. It could be a new parent navigating hemophilia care for their child, or it could be a young adult working to manage their hemophilia care independently.”
While everyone’s experiences with hemophilia are unique, Kyrie’s personal path gives her extra insight into what members of the community are going through.
“When I have the opportunity to connect with a young girl who is having hemophilia symptoms and needs to advocate for herself, I can really relate because I’ve been in her shoes,” Kyrie says. “The road to diagnosis and treatment can be long and confusing, but there are so many reasons to keep going and so many people to help you do just that. I hope by sharing my story, others will too, and together, we can continue empowering the hemophilia community to advocate for their care and supporting those in the earliest stages of their hemophilia journey.”
If you have a family history of hemophilia or you’re experiencing symptoms, you’re not alone. Reach out to your doctor for more information.
To learn more about women and hemophilia, including how it’s inherited, how it presents, and options for treatment, visit RedefiningHemophilia.com.
This is a paid public announcement from Sanofi and does not constitute an endorsement of products or services. When you click on the links in this blog entry, you will be directed to a Sanofi website. LA Kelley Communications always advises you to be a savvy consumer when contacting any company; do not reveal identifying information against your will.
- “Women can have hemophilia, too.” Centers for Disease Control and Prevention, https://www.cdc.gov/ncbddd/hemophilia/features/women-and-hemophilia.html
- “Women and bleeding disorders.” National Hemophilia Foundation, https://www.hemophilia.org/bleeding-disorders-a-z/overview/women-and-bleeding-disorders
- Menorrhagia (heavy menstrual bleeding).” Mayo Clinic, https://www.mayoclinic.org/diseases-conditions/menorrhagia/symptoms-causes/syc-20352829
MAT-US-2303664-v1.0-05/2023