Laurie Kelley
April 30, 2018

Last week the bleeding disorders community met in Cleveland, Ohio at Hemophilia Federation of America‘s annual meeting. It was a fabulous time to meet with friends and colleagues, and to learn about new treatments in inhibitors, new drugs in the pipeline and about psychosocial issues. One of the best attended sessions was the one on gene therapy. Entitled “On the Horizon,” the session was a 90-minute review of new products coming our way, and an overview of gene therapy, how it works and who is working on it.
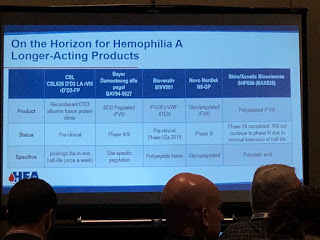
Dr. Sanjay Ahuja, medical director of Rainbow Children’s Hospital, first spoke about “New and Emerging Therapies.” Expression Therapeutics is working on “ET3i,” a recombinant
factor VIII (rFVIII), that should give a higher yield, with the focus on lower cost per unit.
Another interesting therapy is called “transgenic.” Pharming Group has found a way to derive transgenic rFVIII from the milk of rabbits. Ahuja explained that scientists have learned how to take a human gene that makes factor VIII, put in rabbits, and have factor expressed through their milk. This is called “lacto-recombinant factor.”
This generated laughs from the audience, and one man gestured like he was milking a cow. And while Ahuja joked that we could get our kids to drink more milk finally, the actual drug would not be in milk to drink, but commercially available as an infusion. It would be cheaper to produce, with a high yield, making factor much more affordable.
“New things and better things coming,” Ahuja said.
Many people in the audience already knew about the innovative therapy called emicizumab (commercial name: Hemlibra), a bispecific monoclonal antibody that mimics factor VIII by bringing together activated FIX and FX together, replacing the function of FVIIIa. It’s not a factor product! There was a brief discussion about the deaths associated with its use [see our upcoming article in PEN for a detailed discussion on these]. Bioverativ and Shire are also working on bispecific monoclonal antibody and Shire’s is actually a bi/trispecific. These drugs are called “FVIII-Mimetic.”
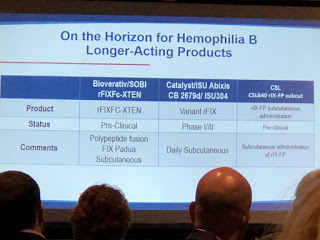
Another innovation for FIX is from Salk Institute/Arcturus Therapeutics, currently in pre-clinical studies. It’s not gene therapy, though it involves taking RNA to the liver to
make factor.
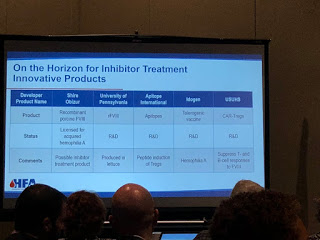
On the horizon for inhibitors are products in the FVII market. HEMA Biologics/LFB, are working on an activated FVII. rEVO Biologics/LFB are working on FVIIa in transgenic rabbits.
Even a long acting, subcutaneous FVIIa is being made by Catalyst Biosciences and OPKP Health.
Perhaps the biggest surprise of all is rFVIII being made in lettuce at the University of Pennsylvania, and this you do eat!
Dr. Stacey Croteau, medical director Boston Children’s Hospital, and Associate Director of the Boston Hemophilia Center next spoke about gene therapy. She gave a brilliant overview, too detailed for here, but if you look at the slides, you’ll get a sense of just how much activity is underway. And all through the four-day conference, I kept hearing chapter leaders talking about not “if” gene therapy occurs, but “when.” More and more, it is becoming a reality.
Dr. Croteau first explained that there are three basic types of gene therapy:
1) Direct therapy (injection into the patient)
2) Cell based (in which you take cells out, alter the genes, then reintroduce the altered cells to the individual, called ex vivo)
3) gene editing (going directly into a defective gene to make it work)
 |
| Branded Content |
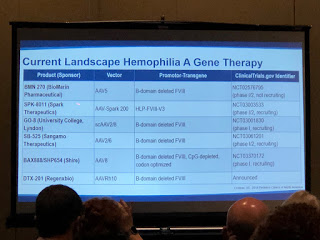
There was a good discussion of how adeno-associated viruses (AAV) are mostly used as the vehicles (vectors) to introduce the altered genes into the patient. Why a virus? They are good at replicating—viruses need to quickly replicate to infect the host and survive. But Dr. Croteau stressed that the AAV8 is stripped down and rendered harmless, so just the FIX gene is left. It’s then introduced back into the patient and goes to the liver (AAV vectors love the liver!), embeds into hepatocytes (liver cells), degrades and becomes part of that cell and starts to express normal coagulation factors into the bloodstream.
Dr. Croteau explained how difficult gene therapy is. You must get the gene delivered to the right cell type in sufficient quantities; then it must switch the gene on, all the time avoiding body’s natural immune response.
In 2011 there was the first successful AAV gene therapy for hemophilia B. With high doses, the patients in the clinical study had their factor levels go from severe to moderate and even to the mild range.
Not all gene therapy research is using AAV; there are all types of AAV vector subtypes. Looking at the slides, why so many? Not everyone will be eligible to use a particular vector. Just like with factor, one gene therapy won’t fit all. Dr. Croteau concluded that it’s good we have several options for gene therapy, and many look very promising!
To learn more about gene therapy trials, you can look at
Clinicaltrials.gov
And very honorably, the speakers reminded us that those patients who have volunteered and are volunteering for new therapies and gene therapy make it possible for the rest of us to enjoy a higher quality of life. Indeed, they are our heroes.
This was a great session to attend; thanks to Drs. Ahuja and Croteau for their presentations! Please read HemaBlog next Sunday when I’ll give an overview of the entire HFA meeting… which was fantastic!