A year ago we renewed a match between two heavy-weight contenders in hemophilia: plasma-derived products versus recombinant. The question: which product is more likely to cause inhibitor formation? In the fight to avoid inhibitor formation, some groups and opinion leaders read the initial results of the SIPPET project and declared plasma-derived the winner. But it’s a bit more complicated than that. Read Paul Clement’s excellent article on SIPPET; it will take many more rounds before we declare an outcome. The good news is that so much clinical scrutiny is underway.
The SIPPET
Bombshell
Paul Clement
A bombshell was dropped at the Plenary Scientific Session of
the 57th annual meeting of the American Society of Hematology (ASH) on December
6, 2015, in Orlando. Study coordinators of the SIPPET project (Study on
Inhibitors in Plasma-Product Exposed Toddlers)1 presented surprising
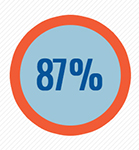
preliminary findings: recombinant factor VIII products are associated with an
87% increased risk of inhibitor development compared to plasma-derived factor
VIII products. 2
In other
words, for every 10 people treated with recombinant factor VIII as opposed to
plasma-derived factor VIII, 1 patient can be expected to develop high-titer
inhibitors.
As a parent
of a toddler who does not have inhibitors, you may feel stunned, angry, or
scared when you read these findings. Should you be? Before you rush to make a
product change, learn how the study was conducted, what its potential
shortfalls are, and why you should take a deep breath!
Shock and Awe
Understandably, many consumers are concerned. Some news
releases describing the study results only heightened the alarm. Hemophilia
Federation of America (HFA) issued a press release requesting that National
Hemophilia Foundation’s (NHF) Medical and Scientific Advisory Council (MASAC)
“consider the temporary suspension of recommendations… that state any
preference for recombinant factor products until the results of the full SIPPET
study can be reviewed.” 3
Is this a
reasonable reaction, or is this jumping the gun? It helps to examine how the
study was conducted—and why.
Fighting Invaders
Why was the study looking to see if plasma-derived products
are less immunogenic than recombinant products—that is, less likely to lead to
developing inhibitors?
In the
blood, factor VIII is normally tightly bound to another protein called von
Willebrand factor (VWF). VWF has several functions, including protecting factor
VIII from being digested and cleared from the bloodstream. Some researchers
suggest that in doing this, VWF masks some of the sites on the factor VIII
protein where antibodies attach, potentially making factor VIII with VWF less
immunogenic. Note:
•
Intermediate/high-purity plasma-derived factor VIII products are the only ones
that contain VWF.
•
Recombinant and ultra-high-purity (monoclonal purified) plasma-derived factor
VIII products contain no VWF.
Without the protection of its VWF “bodyguard,” the immune
system may recognize these factor VIII products as intruders and develop
inhibitors to neutralize them.
The problem is, no one really knows for sure what causes
inhibitors, and no one knows whether factor VIII with VWF is less immunogenic.
SIPPET Strategy
SIPPET set out to answer this question: Is plasma-derived
factor VIII with VWF less immunogenic compared to recombinant factor VIII
without VWF?
SIPPET
researchers designed a study called a prospective
randomized controlled trial (RCT). Prospective means looking forward,
before the patient has developed an inhibitor (in contrast to retrospective studies, in which
researchers look backward, after someone has developed an inhibitor).
Controlled means that there are two groups: (1) an experimental group that will
use factor VIII containing VWF, and (2) a control group that will use factor VIII
without VWF. This second group is used as a standard of comparison against the
experimental group. Randomized means that no one involved in the study
influenced which group a patient was assigned to. Randomization is often done
by a computer.
RCT studies
are often considered the gold standard, thought to produce more reliable data
than other types of studies. Although an RCT can show relationships between
variables being studied, it cannot prove causality. So the RCT used for SIPPET
can’t prove that the presence or absence of VWF in factor VIII caused the observed results.
SIPPET was
conducted between 2010 and 2015, and data was collected on 251 patients from 42
participating sites in 14 countries from Africa, the Americas, Asia, and
Europe. The patients were younger than six years old, had severe hemophilia A,
were previously untreated with factor, and had minimal exposure (less than five
times) to blood components. Of the 251 patients, 125 were treated with one of
the plasma-derived factor VIII products containing VWF. The remaining 126
patients were treated with a VWF-free recombinant factor VIII product.4 The
patients were followed to see if they developed an inhibitor, for 50 exposure
days (days they received factor infusions) or three years, whichever came
first.
It’s
important to note that only one of
the plasma-derived products used in this study is available in the US, and that
the study was funded by manufacturers of plasma-derived products. Is this a
conflict of interest? Does it influence the findings?
SIPPET Shortcomings?
The preliminary findings were startling: of the 251
patients, 76 developed an inhibitor, and 50 of those were high-titer
inhibitors. And 90% of these inhibitors developed in the first 20 days of
treatment. Most important: recombinant factor VIII products were associated
with an 87% increased risk of developing an inhibitor compared to
plasma-derived factor VIII products containing VWF.
Remember, these are not final results and have not yet
been reviewed by researchers outside of the study. Before you decide
whether to switch your toddler to a plasma-derived factor VIII containing VWF,
know that many other variables affect inhibitor formation. In any experiment,
variables not directly being tested, but which could have an effect on the
outcome, are called confounding variables.
For
example, the single greatest risk factor for developing inhibitors is the type
of genetic mutation that caused your child’s hemophilia. If the mutation in the
factor VIII gene resulted in no factor VIII being produced in his body, then he
is already at significantly higher risk of developing an inhibitor. This is one
of many confounding variables in the SIPPET study.
One way to
reduce the effects of confounding variables on the data is to use a large study
sample. If the sample size is large enough and patients are randomly assigned
to two groups, then each group should have about the same number of patients
with the same confounding variable, so its effect will be canceled. The problem
is that the more confounding variables you have, the larger your study sample
size must be—perhaps several thousand patients. And many variables affect
inhibitor development.
Another way
to account for the effects of confounding variables is to identify and measure
them, and then to separately compare and analyze the data from patients who
share the same confounding variable. This process is called stratification (meaning to separate into
layers) and was used by SIPPET along with other statistical analysis methods.
But the study identified and measured only six confounding variables: (1) age
at first treatment, (2) intensity of treatment, (3) type of factor VIII gene
mutation, (4) family history, (5) ethnicity, and (6) country site. What about
the effects of the other confounding variables that were not measured? If the
study sample size was too small to reduce the effects of other, unmeasured,
confounding variables, then the study’s conclusions are questionable and might
be explained in other ways.
Don’t Jump Ship Yet
At the time of this writing, SIPPET has not been published
in a medical journal. That means researchers—outside of those conducting the
study—don’t know much more about the study than you do after reading this
article. Only a short synopsis of the SIPPET study was presented at the ASH
annual meeting—just enough to cause a stir and raise many questions. You can be
sure that as soon as the journal article is released, it will be examined by
bleeding disorder experts worldwide. Questions will undoubtedly be asked about
the handling of confounding variables and whether the study sample size was
large enough.
And experts
will have another question, too: Why didn’t the study include any of the new
prolonged half-life products, several of which appear to have a lower
immunogenicity than other recombinant factor VIII products?
Should you
switch your toddler from a recombinant to a plasma-derived factor VIII product
containing VWF based on the preliminary SIPPET results, in the hope that it
will reduce the risk of developing an inhibitor? This is a question for you and
your hematologist, but if you were a betting person, the answer would be no. To
bleeding disorder experts, the results of SIPPET are not a bombshell, but
merely a piece of the puzzle that is inhibitors.5 The conclusions of this study
contradict those of several other studies. It may take years, and several
additional studies, to sort everything out. MASAC is on top of this, and as the
data becomes available, you can be assured that NHF will share its expert
opinion. So keep calm and carry on!
1.
https://ash.confex.com/ash/2015/webprogram/Paper82866.html (accessed Feb. 7,
2016).
2. Inhibitors are
a major complication of hemophilia in which a person’s immune system mistakenly
recognizes infused factor as a foreign (and potentially dangerous) protein, and
develops antibodies (inhibitors) to inactivate the factor, making factor
infusions ineffective.
3.
http://www.hemophiliafed.org/news-stories/2015/12/update-2-sippet-study-2/
(accessed Feb. 7, 2016).
4. The VWF-rich
plasma-derived factor VIII concentrates used by SIPPET: Alphanate (Grifols),
Fandhi (Grifols), Emoclot (Kedrion), or Factane (LFB). The VWF-free recombinant
factor VIII products used: Recombinate (Baxalta), Advate (Baxalta), Kogenate SF
(Bayer), or Refacto AF (Pfizer).
5. Visit the
Believe Limited website for an excellent interview by Patrick James Lynch of
bleeding disorder expert Dr. Steven Pipe about the SIPPET findings:
http://believeltd.com/inhibitors-sippet-and-the-double-edged-internet/
(accessed Feb. 7, 2016).