December 2016
Plasma-Derived vs Recombinant
Bombshell
the 57th annual meeting of the American Society of Hematology (ASH) on December
6, 2015, in Orlando. Study coordinators of the SIPPET project (Study on
Inhibitors in Plasma-Product Exposed Toddlers)1 presented surprising
preliminary findings: recombinant factor VIII products are associated with an
87% increased risk of inhibitor development compared to plasma-derived factor
VIII products. 2
words, for every 10 people treated with recombinant factor VIII as opposed to
plasma-derived factor VIII, 1 patient can be expected to develop high-titer
inhibitors.
of a toddler who does not have inhibitors, you may feel stunned, angry, or
scared when you read these findings. Should you be? Before you rush to make a
product change, learn how the study was conducted, what its potential
shortfalls are, and why you should take a deep breath!
releases describing the study results only heightened the alarm. Hemophilia
Federation of America (HFA) issued a press release requesting that National
Hemophilia Foundation’s (NHF) Medical and Scientific Advisory Council (MASAC)
“consider the temporary suspension of recommendations… that state any
preference for recombinant factor products until the results of the full SIPPET
study can be reviewed.” 3
reasonable reaction, or is this jumping the gun? It helps to examine how the
study was conducted—and why.
are less immunogenic than recombinant products—that is, less likely to lead to
developing inhibitors?
blood, factor VIII is normally tightly bound to another protein called von
Willebrand factor (VWF). VWF has several functions, including protecting factor
VIII from being digested and cleared from the bloodstream. Some researchers
suggest that in doing this, VWF masks some of the sites on the factor VIII
protein where antibodies attach, potentially making factor VIII with VWF less
immunogenic. Note:
Intermediate/high-purity plasma-derived factor VIII products are the only ones
that contain VWF.
Recombinant and ultra-high-purity (monoclonal purified) plasma-derived factor
VIII products contain no VWF.
system may recognize these factor VIII products as intruders and develop
inhibitors to neutralize them.
inhibitors, and no one knows whether factor VIII with VWF is less immunogenic.
factor VIII with VWF less immunogenic compared to recombinant factor VIII
without VWF?
researchers designed a study called a prospective
randomized controlled trial (RCT). Prospective means looking forward,
before the patient has developed an inhibitor (in contrast to retrospective studies, in which
researchers look backward, after someone has developed an inhibitor).
Controlled means that there are two groups: (1) an experimental group that will
use factor VIII containing VWF, and (2) a control group that will use factor VIII
without VWF. This second group is used as a standard of comparison against the
experimental group. Randomized means that no one involved in the study
influenced which group a patient was assigned to. Randomization is often done
by a computer.
are often considered the gold standard, thought to produce more reliable data
than other types of studies. Although an RCT can show relationships between
variables being studied, it cannot prove causality. So the RCT used for SIPPET
can’t prove that the presence or absence of VWF in factor VIII caused the observed results.
conducted between 2010 and 2015, and data was collected on 251 patients from 42
participating sites in 14 countries from Africa, the Americas, Asia, and
Europe. The patients were younger than six years old, had severe hemophilia A,
were previously untreated with factor, and had minimal exposure (less than five
times) to blood components. Of the 251 patients, 125 were treated with one of
the plasma-derived factor VIII products containing VWF. The remaining 126
patients were treated with a VWF-free recombinant factor VIII product.4 The
patients were followed to see if they developed an inhibitor, for 50 exposure
days (days they received factor infusions) or three years, whichever came
first.
important to note that only one of
the plasma-derived products used in this study is available in the US, and that
the study was funded by manufacturers of plasma-derived products. Is this a
conflict of interest? Does it influence the findings?
patients, 76 developed an inhibitor, and 50 of those were high-titer
inhibitors. And 90% of these inhibitors developed in the first 20 days of
treatment. Most important: recombinant factor VIII products were associated
with an 87% increased risk of developing an inhibitor compared to
plasma-derived factor VIII products containing VWF.
been reviewed by researchers outside of the study. Before you decide
whether to switch your toddler to a plasma-derived factor VIII containing VWF,
know that many other variables affect inhibitor formation. In any experiment,
variables not directly being tested, but which could have an effect on the
outcome, are called confounding variables.
example, the single greatest risk factor for developing inhibitors is the type
of genetic mutation that caused your child’s hemophilia. If the mutation in the
factor VIII gene resulted in no factor VIII being produced in his body, then he
is already at significantly higher risk of developing an inhibitor. This is one
of many confounding variables in the SIPPET study.
reduce the effects of confounding variables on the data is to use a large study
sample. If the sample size is large enough and patients are randomly assigned
to two groups, then each group should have about the same number of patients
with the same confounding variable, so its effect will be canceled. The problem
is that the more confounding variables you have, the larger your study sample
size must be—perhaps several thousand patients. And many variables affect
inhibitor development.
to account for the effects of confounding variables is to identify and measure
them, and then to separately compare and analyze the data from patients who
share the same confounding variable. This process is called stratification (meaning to separate into
layers) and was used by SIPPET along with other statistical analysis methods.
But the study identified and measured only six confounding variables: (1) age
at first treatment, (2) intensity of treatment, (3) type of factor VIII gene
mutation, (4) family history, (5) ethnicity, and (6) country site. What about
the effects of the other confounding variables that were not measured? If the
study sample size was too small to reduce the effects of other, unmeasured,
confounding variables, then the study’s conclusions are questionable and might
be explained in other ways.
in a medical journal. That means researchers—outside of those conducting the
study—don’t know much more about the study than you do after reading this
article. Only a short synopsis of the SIPPET study was presented at the ASH
annual meeting—just enough to cause a stir and raise many questions. You can be
sure that as soon as the journal article is released, it will be examined by
bleeding disorder experts worldwide. Questions will undoubtedly be asked about
the handling of confounding variables and whether the study sample size was
large enough.
will have another question, too: Why didn’t the study include any of the new
prolonged half-life products, several of which appear to have a lower
immunogenicity than other recombinant factor VIII products?
switch your toddler from a recombinant to a plasma-derived factor VIII product
containing VWF based on the preliminary SIPPET results, in the hope that it
will reduce the risk of developing an inhibitor? This is a question for you and
your hematologist, but if you were a betting person, the answer would be no. To
bleeding disorder experts, the results of SIPPET are not a bombshell, but
merely a piece of the puzzle that is inhibitors.5 The conclusions of this study
contradict those of several other studies. It may take years, and several
additional studies, to sort everything out. MASAC is on top of this, and as the
data becomes available, you can be assured that NHF will share its expert
opinion. So keep calm and carry on!
https://ash.confex.com/ash/2015/webprogram/Paper82866.html (accessed Feb. 7,
2016).
a major complication of hemophilia in which a person’s immune system mistakenly
recognizes infused factor as a foreign (and potentially dangerous) protein, and
develops antibodies (inhibitors) to inactivate the factor, making factor
infusions ineffective.
http://www.hemophiliafed.org/news-stories/2015/12/update-2-sippet-study-2/
(accessed Feb. 7, 2016).
plasma-derived factor VIII concentrates used by SIPPET: Alphanate (Grifols),
Fandhi (Grifols), Emoclot (Kedrion), or Factane (LFB). The VWF-free recombinant
factor VIII products used: Recombinate (Baxalta), Advate (Baxalta), Kogenate SF
(Bayer), or Refacto AF (Pfizer).
Believe Limited website for an excellent interview by Patrick James Lynch of
bleeding disorder expert Dr. Steven Pipe about the SIPPET findings:
http://believeltd.com/inhibitors-sippet-and-the-double-edged-internet/
(accessed Feb. 7, 2016).
See how James found the factor IX option he’d been searching for
share this inspiring story of a young man with hemophilia B named James. Please read below to learn about his
story and his choice of treatment.
Watch James share his story at IXINITY.com.
Interested in learning more about IXINITY? Connect with a Hemophilia Territory Manager near you at MyIXINITYRep.com.
And remember, this is James’ experience; different people may have different results. You should always talk to your doctor to decide which treatment is right for you.
Meet James
“Since birth I’ve used different hemophilia B products, and regardless of treatment, I continued to have breakthrough bleeds most every other week. I felt I had no control over my health, and that led me down a bad path as I started making some unhealthy choices in my teen years.
When I was 21, I saw an ad for a clinical trial of a new recombinant hemophilia B treatment. I thought, ‘I have nothing to lose.’
After a few weeks in the clinical trial, I stopped having bleeds almost entirely. I realized I didn’t know what feeling good felt like. I felt more in control, and it was a liberating experience.
One day I decided to go running—I don’t know what made me decide to because I’d never really done it before. But I thought, ‘I’m just going to go for a run,’ and about a mile later, I thought, ‘Huh! This is awesome’
After talking with my doctor, I’ve now developed a habit of running 3 times each week with my dog, and exercising regularly.
To be honest, switching to IXINITY was the best decision I ever made. I don’t feel like someone with hemophilia; I forget I even have it.”
See more of James’ journey at IXINITY.com.
James’ experience with IXINITY may not be typical. Speak with your doctor to see if IXINITY is the right treatment for you.
The content of this post is provided and sponsored by Aptevo Therapeutics.
IXINITY INDICATIONS AND IMPORTANT SAFETY INFORMATION
What is IXINITY®?
IXINITY [coagulation factor IX (recombinant)] is a medicine used to replace clotting factor (factor IX) that is missing in adults and children at least 12 years of age with hemophilia B. Hemophilia B is also called congenital factor IX deficiency or Christmas disease. Hemophilia B is an inherited bleeding disorder that prevents clotting. Your healthcare provider may give you IXINITY to control and prevent bleeding episodes or when you have surgery.
IXINITY is not indicated for induction of immune tolerance in patients with hemophilia B.
IMPORTANT SAFETY INFORMATION for IXINITY®
• You should not use IXINITY if you are allergic to hamsters or any ingredients in IXINITY.
• You should tell your healthcare provider if you have or have had medical problems, take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies, have any allergies, including allergies to hamsters, are nursing, are pregnant or planning to become pregnant, or have been told that you have inhibitors to factor IX.
• You can experience an allergic reaction to IXINITY. Contact your healthcare provider or get emergency treatment right away if you develop a rash or hives, itching, tightness of the throat, chest pain, or tightness, difficulty breathing, lightheadedness, dizziness, nausea, or fainting.
• Your body may form inhibitors to IXINITY. An inhibitor is part of the body’s defense system. If you develop inhibitors, it may prevent IXINITY from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for development of inhibitors to IXINITY.
• If you have risk factors for developing blood clots, the use of IXINITY may increase the risk of abnormal blood clots.
• Call your healthcare provider right away about any side effects that bother you or do not go away, or if your bleeding does not stop after taking IXINITY.
• The most common side effect that was reported with IXINITY during clinical trials was headache.
• These are not all the side effects possible with IXINITY. You can ask your healthcare provider for information that is written for healthcare professionals.
For more information about IXINITY, please see full Prescribing Information, including Important Patient Information.
You are encouraged to report side effects of prescription drugs to the Food and Drug Administration. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Aptevo BioTherapeutics LLC, Berwyn, PA 19312
IXINITY [coagulation factor IX (recombinant)] and any and all Aptevo BioTherapeutics LLC brand, product, service and feature names, logos, and slogans are trademarks or registered trademarks of Aptevo BioTherapeutics LLC in the United States and/or other countries.
© 2016 Aptevo Biotherapeutics. All rights reserved. CM-FIX-0093
AIDS: Lest We Forget
 |
| “Get out!” |
The threat of HIV to those with hemophilia was made vividly clear in September 1987. On August 21, 1987, two weeks before my son with hemophilia was born, the Ray brothers had been fire-bombed from their trailer park home in Arcadia, Florida–a warning to the family to leave. It made headlines nationwide and was considered a landmark act in the history of HIV in the US. The story was of shocking interest to everyone, even those of us who didn’t know hemophilia was about to enter our lives.
 I still have my copy of People magazine, which had a story on it. Two weeks later, my son was diagnosed and a chill went through me. He was lucky to have just missed the window for contracting HIV through contaminated blood products. But there was national hysteria, misunderstanding about HIV transmission, and paranoia.
I still have my copy of People magazine, which had a story on it. Two weeks later, my son was diagnosed and a chill went through me. He was lucky to have just missed the window for contracting HIV through contaminated blood products. But there was national hysteria, misunderstanding about HIV transmission, and paranoia.
World AIDS Day remembers those who died, and a ceremony was held at the National AIDS Memorial Grove in San Francisco. Both HFA and NHF were honored to have been invited to participate.
http://www.aidsmemorial.org/
World AIDS Day also serves to remind us that the work is not yet done. I was reading Time magazine on a plane ride yesterday, and was shocked at the statistics I read.
- In 2015, 39,513 people were diagnosed with HIV infection in the United States.
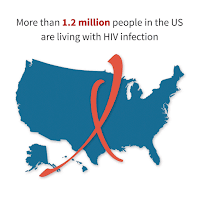
- More than 1.2 million people in the US are living with HIV, and 1 in 8 of them don’t know it.
- Gay and bisexual men accounted for 82% (26,375) of HIV diagnoses among males and 67% of all diagnoses.
- Black/African American gay and bisexual men accounted for the largest number of HIV diagnoses (10,315), followed by white gay and bisexual men (7,570).
- Heterosexual contact accounted for 24% (9,339) of HIV diagnoses
- Six percent (2,392) of HIV diagnoses in the United States were attributed to injection drug use (IDU).
- Louisiana has the second highest new-infection rate: due to poverty, large incarcerated population, stigma, abstinence-only sex ed (CDC data, Time magazine, Nov 28-Dec 5, 2016)
- Miami: cuts in health spending contributed to a 23% rise in those with HIV since 2004, the fastest-growing rate of infection in US. (CDC data, Time magazine, Nov 28-Dec 5, 2016)
Note: The spread of HIV in the US was originally blamed on a gay French Canadian flight attendant, Gaetan Dugas, but new analysis of stored blood samples have exonerated him of this. And the Band Played On opens with Dugas’s story and alleged link as Patient Zero.







