It was bound to happen sooner or later: in a community flush with hemophilia A therapies, one of them would have to give.
This past week Bayer announced that it would discontinue production of Kogenate® FS (Antihemophilic Factor (Recombinant), which has been in use since 1993. I’m nostalgic about it leaving; when it appeared, my second born was only 3. We used it at one point. We educated the community that it was the exact same product as Helixate®FS… though many parents tried to argue with me that they had different names and were from different companies, and were therefore different. They were not.
But times are truly changing. We have extended half-life (“long lasting”) products now; we have products made from a human cell line, and not hamster cell lines. We even have transgenic animal therapies. And we have Hemlibra, an injectable with a half-life of over 600 hours.
But above all, we have probably too many factor products for hemophilia A patients, who number around 20,000 in the US. How will the market justify all the products?
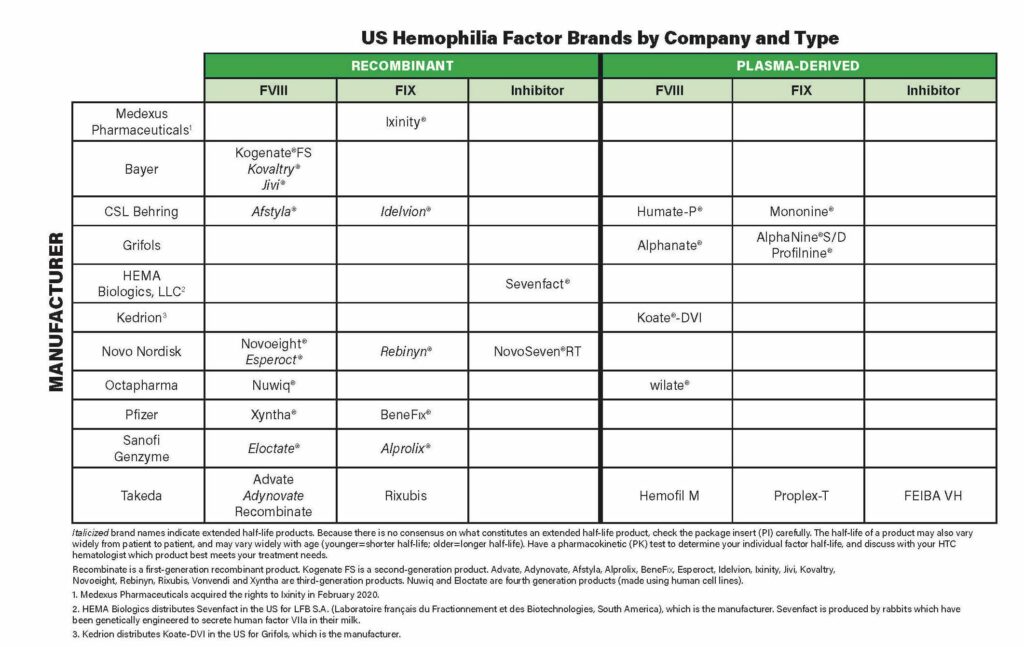
Our factor chart below shows 12 products for hemophilia A that are recombinant, and five products that are plasma-derived (from human blood). Some manufacturers, like Bayer, Novo Nordisk and Takeda, have multiple factor VIII products. It’s like they are in competition with themselves. Some are standard factor products and others are extended half-life.
Bayer’s press release says: “Recognizing the growing shift in patient use toward more recent products, such as Kovaltry® (Antihemophilic Factor (Recombinant)) and Jivi® (Antihemophilic Factor (Recombinant), PEGylated-aucl), Bayer has made the decision to discontinue Kogenate® FS (Antihemophilic Factor (Recombinant)) in the United States.
“Keeping the needs of patients in mind, Bayer is keenly aware that they will need sufficient time to work with their HCP to make decisions about their next treatment. The timing of discontinuation will vary by Kogenate FS vial size. Customer demand may lead to depletion of the larger vial sizes of Kogenate FS during the fall of 2022. Remaining Kogenate FS vial sizes are anticipated to be available into 2023.
“As Kogenate FS patients and their caregivers embark on the next step of their journey, Bayer is committed to supporting them. Attached, please find our discontinuation announcement for your reference. Additionally, we have set up a website (explore.bayer.com) and a dedicated Kogenate FS hotline for anyone who has questions regarding this discontinuation, (1-833-40-BAYER), which is available Monday – Friday, 8:30am – 8:00pm ET.” See the full press release here.
If you are a Kogenate FS user, it’s time to contact your HTC staff and discuss next steps. We’ve been through this before over the decades: we know that some patients want to stay with the manufacturer, and will switch to their other products. Other patients will take this as an opportunity to learn more about other products from other manufacturers. Whichever you are, be sure you make your decision with your HTC staff… not the internet! Not even with me. Call the manufacturer and speak with your HTC staff, learn more about these products, and then choose which is right for you.
And probably more change is coming, in products, manufacturers… and eventually gene therapy.
Resolve to be a master of change rather than a victim of change.— Brian Tracy

