New Publication Answers Questions About the SIPPET Study
Inhibitors are the most troubling complication of hemophilia A treatment today. In this week’s blog, I share with you a new publication concerning the SIPPET study, which sheds light on factor VIII products and inhibitors.
- Is the inhibitor risk higher in SIPPET than in previous reports?
- Could differences in treatments between countries have affected the results?
- Could the results have been affected by the way the study was randomized?
- Do the SIPPET results also apply to other recombinant factor VIII (rFVIII) products
beyond the 1st and 2nd generation products used in the study? - Is there a difference in inhibitor risk between the different brands within the plasmaderived and recombinant groups?
| Original SIPPET Study The SIPPET study, conducted by Dr. Peyvandi and colleagues, was the first randomized trial to compare the incidence of inhibitors in plasma-derived factor VIII (pdFVIII/VWF) products and rFVIII products in previously untreated patients (PUPs).2 |
|
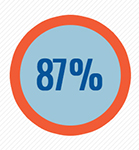
| Results from this landmark study showed that there was an 87% higher rate of inhibitor development in patients who received rFVIII compared with patients who received pdFVIII containing von Willebrand factor (VWF).2 | |
| Based on the results of the SIPPET study, the National Hemophilia Foundation’s Medical and Scientific Advisory Council (MASAC) now recommends that pdFVIII/VWF be considered as one of several treatment options in PUPs.3 |

|
Visit www.inhibitorinfo.com to Learn More About Inhibitors Inhibitors are the most serious and challenging hemophilia A treatment complications. All patients with hemophilia A are at risk for developing inhibitors, regardless of age and disease severity. |
| Inhibitorinfo.com is a comprehensive website that provides important information and resources about inhibitors and the risk of inhibitors. There is a discussion guide patients can download and use to talk with their hematologists about inhibitors. Visitors can also read about the results of the SIPPET study and watch leading hematologists talk about its implications. |
When visitors sign up for updates at inhibitorinfo.com, they will receive access to the full SIPPET study, as well as updates about hemophilia, inhibitors, and the latest clinical data.
References: 1. Peyvandi F, Mannucci PM, Palla R, Rosendaal FR. SIPPET: methodology, analysis
and generalizability [published online ahead of print March 17, 2017]. Haemophilia. doi:
10.1111/hae.13203. 2. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII
and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064. 3. National
Hemophilia Foundation. MASAC Update on SIPPET. National Hemophilia Foundation website.
https://www.hemophilia.org/Newsroom/NHF-Community-News/MASAC-Update-on-SIPPET.
Published March 9, 2016. Accessed April 27, 2017.
and generalizability [published online ahead of print March 17, 2017]. Haemophilia. doi:
10.1111/hae.13203. 2. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII
and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064. 3. National
Hemophilia Foundation. MASAC Update on SIPPET. National Hemophilia Foundation website.
https://www.hemophilia.org/Newsroom/NHF-Community-News/MASAC-Update-on-SIPPET.
Published March 9, 2016. Accessed April 27, 2017.
This is a paid public announcement from Grifols and does not constitute an endorsement of
products or services. When you click on the links in this blog entry, you will be directed to
the Grifols website. LA Kelley Communications always advises you to be a savvy consumer
when contacting any company; do not reveal identifying information against your will.
products or services. When you click on the links in this blog entry, you will be directed to
the Grifols website. LA Kelley Communications always advises you to be a savvy consumer
when contacting any company; do not reveal identifying information against your will.
BN/A8/0517/0275