1,047
This week marks the first ever International Plasma Awareness Week, an opportunity to recognzie the importance of plasma in our lives, and the need to donate blood to provide the life-saving liquid to those in need. It’s especially important to many with hemophilia throughout the world as donated blood donations can be fractionated into factor VIII and IX for many hemophilia patients who use plasma therapy.
You learned this in high school science class but here it is again: Plasma is the clear, straw-colored liquid portion of blood that remains after red blood cells, white blood cells, platelets and other cellular components are removed. It is the single largest component of human blood, comprising about 55 percent, and contains water, salts, enzymes, antibodies and other proteins. It is a clear, straw colored liquid that is 90% water and serves as a transporting medium for cells and a variety of substances vital to the human body.
In the US, we often think only of recombinant factor when we think about treating hemophilia, but plasma therapies remain the mainstay for many with hemophilia, those with inhibitors and undergoing ITI, and those with von Willebrand disease patients.
 In addition, plasma protein therapies are used to in emergency and surgical medicine.Plasma protein therapies are not interchangeable and have been defined by regulators as sole-source biologic products because no generics or substitutions exist. In addition, their biological nature demands storage and handling requirements by specialty distributors that ensure their safety.
In addition, plasma protein therapies are used to in emergency and surgical medicine.Plasma protein therapies are not interchangeable and have been defined by regulators as sole-source biologic products because no generics or substitutions exist. In addition, their biological nature demands storage and handling requirements by specialty distributors that ensure their safety.
Source plasma is plasma that is collected exclusively for further manufacturing through a process called plasmapherisis. Recovered plasma is collected through whole blood donation which has been separated into its cellular components.
Safety and quality of plasma protein therapies is the top priority of the plasma protein therapeutics industry. Both collectors and manufacturers adhere to strict regulatory policies and have instituted Good Manufacturing Practices in every step of plasma collection and manufacturing processes.
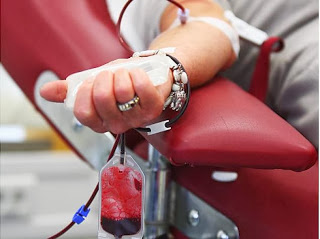
Plasma-derived therapies depend on the generosity and commitment of healthy donors. Source plasma is collected in over 450 specialized donor centers in the U.S., Canada, Germany, Austria and the Czech Republic. Source plasma collection in the U.S. is regulated by the Food and Drug Administration and by the European Medicines Agency and national regulatory authorities in Europe. Additionally, 436 plasma collection centers are also certified by the International Quality Plasma Program (IQPP), a rigorous, voluntary program that goes beyond regulatory requirements to help ensure donor safety and further improve the quality of plasma used to manufacture therapies.
This is a good week to donate blood then! If you can make the time, please visit your local blood donation center and give the gift of life.
See www.donatingplasma.org and www.pptaglobal.org for more information.


