A Personal Approach to Healthcare
Ever hear of personalized healthcare (PHC)? PHC means tailoring a treatment regimen specific to an individual patient. It acknowledges that every patient is different, with different physiology, biology, reactions to medicine, and lifestyle. A treatment regimen that works for one patient may not work for another with the exact same ailment.
The goal of PHC is to improve your quality of life as a patient with a specific disease or disorder, by taking into
account your individual needs and lifestyle, and then tailoring a treatment plan. How does that happen? It’s a collaborative process involving you and your medical team. Your HTC medical team knows all the treatment options, but you know yourself, your body, and your lifestyle. To develop your own PHC, you’ll need to share with the team every aspect of your past health, current health, needs, and desires. Only then can you all explore treatment options that best suit you.
Your Personalized Healthcare Team
The best place to get help with hemophilia and all bleeding disorders is at a hemophilia treatment center (HTC). These are centers of excellence that specialize in diagnosing, treating, and monitoring bleeding disorders. There are over 140 HTCs throughout the US. They follow the model of comprehensive healthcare, which means that they’re not just treating bleeding episodes, but all aspects of living with a bleeding disorder. From orthopedics to psychosocial needs to genetics, the HTC team knows how a bleeding disorder can impact your life.
By using comprehensive healthcare, isn’t your HTC team already implementing PHC?
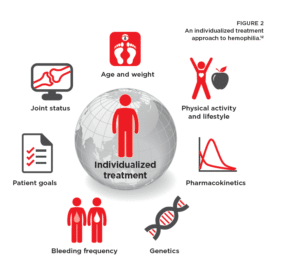
Maybe not. First, not all HTCs are created equal. Some may lack certain experts you need, such as a pediatric hematology department or a geneticist. And some may have strong beliefs, different from yours, about when—or whether—to start prophylaxis (prophy), or about whether you should try new products.
Parents and patients need to become partners in PHC. Are they ready for this collaboration? Parents of newly
diagnosed children may be too shocked at first, and not even know what questions to ask. Older patients may be overlooked for PHC because they’ve been on the same treatment plan for a long time, their blood work is good, and they don’t complain or ask questions.
It’s important for you to be ready to partner with your HTC and let the team know your needs. But even if you’re comfortable standing up and being heard, what will you say? What will you ask?
1. CHOOSE THE RIGHT TREATMENT
One of the first decisions you need to make about your PHC is which treatment to use. All products licensed in the US are considered safe and effective, but they’re not all the same. How do you find one that’s best for you?
You know that new products are entering the market. You can choose between plasma-derived (made from human blood) and recombinant (made from animal cells containing human genes). Within the recombinant products, there are categories: first, second, or third generation. And there are novel therapies, that are not even factor! So begin by asking your hematologist for opinions on all products.
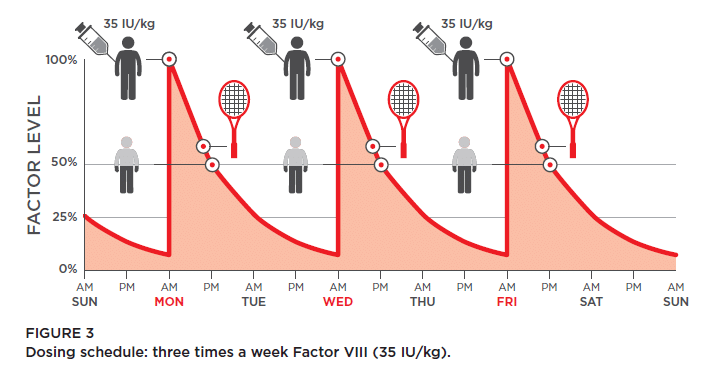
Your decision about treatment may come down to lifestyle, when selecting a particular brand for prophy. Perhaps the brand you’re using now, three times a week, works well for you or your child. Does a product with an extended half-life offer fewer infusions, saving veins from wear and tear? Or would you benefit from a new, subcutaneous product? Some products might not be available through your insurance. Talk through these choices with your HTC.
2. BLEEDING PATTERNS
Perhaps nothing is more personal than your individual bleeding pattern. People with hemophilia bleed differently, in different places, from different causes. Once your child begins getting bleeds, notice his bleeding pattern, if any. This is where you can really help your HTC team personalize your treatment. The information you share can help prevent a target joint from starting, or can compel the HTC team to put your child on prophy.
You may hear severity levels described like this: Children with severe hemophilia will bleed from trauma, or spontaneously, with no known trauma. Children with moderate hemophilia may bleed on average once a month, with known trauma. Children with mild hemophilia may bleed only after dental extractions and surgery. But what’s the reality? Some children with severe hemophilia bleed only monthly; some with moderate bleed every week, with no known trauma. Your child’s bleeding is unique!
And know his personal symptoms of a bleed: Tingling? Hot? Swollen? Your HTC staff can help you identify symptoms, so you can infuse more quickly at the first sign of a bleed.
3. ESTABLISH A TREATMENT REGIMEN
Personalized healthcare really shines when it’s time to devise a treatment plan. You can use the new, subcutaneous novel therapy. Or you have two options for infusing factor: on-demand (also called episodic) or prophylaxis. On-demand means infusing at the first sign of a bleed. Prophy is the scheduled infusing of factor. It’s designed to keep factor levels in the bloodstream high enough—greater than 1%—to prevent most spontaneous bleeds.
Prophy is the recommended therapy for children with hemophilia in countries like the US with ready access to clotting factor.
And what about your schedule for prophy? This is about as personal as PHC gets! Your HTC team will offer a schedule based on your child’s needs: your family lifestyle, activity level, perhaps pharmacokinetics (PK) data to determine how quickly factor is cleared from the blood after an infusion, and any breakthrough bleeding that might happen.
4. DISCUSS PERSONALIZED MEDICINE
Personalized healthcare is not the same as personalized medicine, a common term. Personalized medicine often refers to using a person’s genetic profile—genomes and specific genetic markers—to guide therapy for cancer and other diseases and disorders, including hemophilia. But PHC includes personalized medicine, and it’s worth discussing with your HTC team because more and more, the hemophilia community is focusing on personalized medicine.
National Hemophilia Foundation (NHF) recognized the importance of this genetic research and launched My Life, Our Future (MLOF). Through this program, you can get a blood test that enables you to learn more about the specific genetic mutation that caused your child’s hemophilia.
So PHC uses personalized medicine to examine your genetic makeup, help predict which medical disorders or diseases your child is most at risk for, and suggest which treatments will be safe and effective (or not) for him. This is particularly important for complications like the risk of getting inhibitors.
We’ve shown you examples of treatment and lifestyle areas to focus on, potential needs to address, and questions to ask your HTC team to design the best life possible with your bleeding disorder. Your HTC team will become one of your most important partners.






 In the years that followed, we advanced the treatment of hemophilia A, hemophilia B, hemophilia A or B with inhibitors, von Willebrand disease, and acquired hemophilia A with significant developments. Many of these were firsts: the first recombinant factor VIII treatment, the first needleless transfer device, the first recombinant factor VIII treatment free of blood-based additives, the first recombinant treatment for people with von Willebrand disease, and the first recombinant porcine factor VIII for acquired hemophilia.6-9
In the years that followed, we advanced the treatment of hemophilia A, hemophilia B, hemophilia A or B with inhibitors, von Willebrand disease, and acquired hemophilia A with significant developments. Many of these were firsts: the first recombinant factor VIII treatment, the first needleless transfer device, the first recombinant factor VIII treatment free of blood-based additives, the first recombinant treatment for people with von Willebrand disease, and the first recombinant porcine factor VIII for acquired hemophilia.6-9 Thanks to the many contributions that have been made in the past, and which Shire continues to make, to the treatment of bleeding disorders, Shire’s vision for patients with a bleeding disorder is closer to realization than ever before.
Thanks to the many contributions that have been made in the past, and which Shire continues to make, to the treatment of bleeding disorders, Shire’s vision for patients with a bleeding disorder is closer to realization than ever before. And the innovation continues. Research and development is going strong with 20 ongoing clinical trials in bleeding disorders, including one in gene therapy, as well as advancements in other novel therapies. Shire has engaged hundreds of the world’s leading scientists, researchers, and patient support specialists to help them.5
And the innovation continues. Research and development is going strong with 20 ongoing clinical trials in bleeding disorders, including one in gene therapy, as well as advancements in other novel therapies. Shire has engaged hundreds of the world’s leading scientists, researchers, and patient support specialists to help them.5 Most fundamentally, Shire is collaborating with the bleeding disorders community, including patient associations that have enabled the diagnosis of more than 30,000 hemophilia patients around the world.5 Shire has listened to, learned from, and championed their needs. This bleeding disorders community is our community. It’s why Shire is always pushing ahead, proactively shaping the future of bleeding disorders and continually elevating care for patients.
Most fundamentally, Shire is collaborating with the bleeding disorders community, including patient associations that have enabled the diagnosis of more than 30,000 hemophilia patients around the world.5 Shire has listened to, learned from, and championed their needs. This bleeding disorders community is our community. It’s why Shire is always pushing ahead, proactively shaping the future of bleeding disorders and continually elevating care for patients.