This is a paid public announcement from Sanofi and does not constitute an endorsement of products or services. When you click on the links in this blog entry, you will be directed to a Sanofi website. LA Kelley Communications always advises you to be a savvy consumer when contacting any company; do not reveal identifying information against your will.
If your life has been touched by hemophilia A, you know it can be full of unknowns. Bleeds can be painful, happen without warning, and limit physical activity, which is why it’s critical to stay on top of them. Sanofi launched ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl]—a hemophilia A Factor VIII replacement therapy that provides proven bleed protection.
Here are 4 reasons to consider ALTUVIIIO:
1. HIGHER FACTOR LEVELS THAT LAST LONGER
With just one weekly ALTUVIIIO infusion, factor levels remain in the near-normal to normal range (>40%) for most of the week and stay above 18%,* on average, in adults.
*Average trough levels were 18% for adults 18 years and older, 9% for adolescents aged 12 years to under 18 years, 10% for children aged 6 years to under 12 years, and 7% for children aged 1 year to under 6 years.
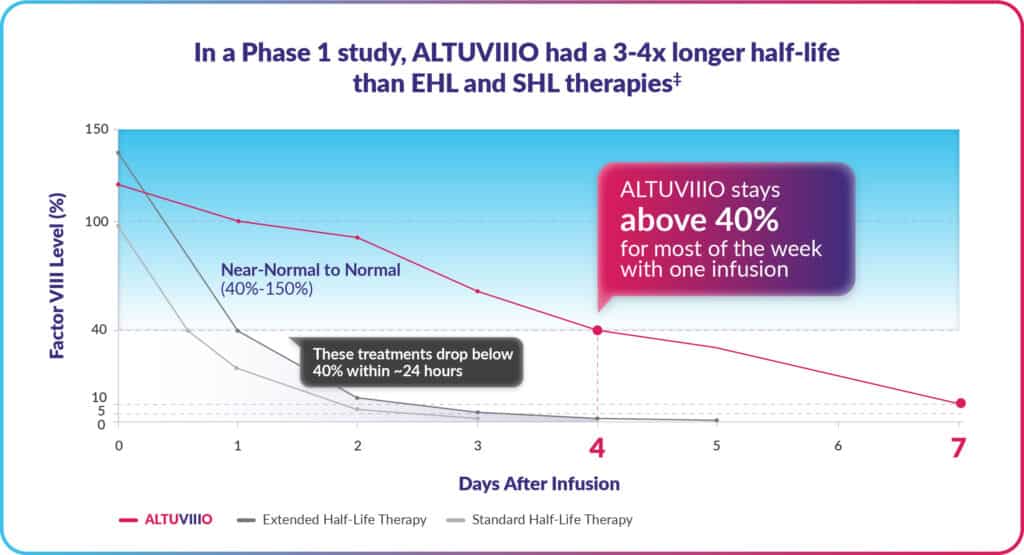
‡This is information from a study of 13 previously treated adults with severe hemophilia A that had the goal of comparing how long ALTUVIIIO, Adynovate® [Antihemophilic Factor (Recombinant), PEGylated], and Advate® [Antihemophilic Factor (Recombinant)] stayed in the body after 1 dose. Half-life was 43 hours for ALTUVIIIO, 15 hours for Adynovate, and 11 hours for Advate.
Adynovate and Advate are registered trademarks of Baxalta Incorporated, a Takeda company.
ALTUVIIIO offers not only weekly prophylaxis use, but also on-demand bleed control and perioperative management. Regardless of how you use it, you can expect the same infusion process.
2. STUDIED—AND PROVEN—BLEED PROTECTION
Before we dig into the numbers, it’s helpful to know how ALTUVIIIO was studied and to understand its safety profile.
For one year, the XTEND-1 study looked at treatment in 159 adults and adolescents. Participants were divided into 2 groups. Both groups used mean and median annualized bleed rates (ABRs) to evaluate the efficacy of ALTUVIIIO. Finding people’s mean ABR was the primary goal of the study.
Safety evaluated in 159 people taking ALTUVIIIO in the XTEND-1 study showed that:
- 21% of people had headache (33 people)
- 16% of people had joint pain (26 people)
- 6% of people had back pain (9 people)
In 67 children taking ALTUVIIIO in the ongoing XTEND-Kids study:
- 1% of children had headache (1 child)
In XTEND-1 and XTEND-Kids, people taking ALTUVIIIO had:
- 0 inhibitors
- 0 serious allergic reactions
Although no inhibitors were found, and no serious allergic reactions occurred in clinical studies, inhibitors and serious allergic reactions are possible with ALTUVIIIO.
Group 1
This group consisted of 133 people aged 12 years and older who had prior prophylaxis therapy and switched to ALTUVIIIO weekly prophylaxis. This group included 1 female participant. Efficacy of prophylaxis was evaluated in 128 of these patients.
The primary outcome showed a mean of <1 (0.7) bleeds per year (the median ABR was 0).‡
Here’s a look at how the study measured bleed rates:
- Median ABR was the middle number of all ABRs, when ABRs were ordered from least to greatest
- Mean ABR was the average number based on everyone’s ABRs
It’s also worth noting that 78 of the people in Group 1 participated in a separate study to measure their ABRs on their prior prophylaxis. These 78 people went from 3 bleeds to less than 1 bleed a year. That’s a 77% reduction in yearly bleeds!‡
Group 2
People in this group (28 participants) switched to ALTUVIIIO on demand from prior on-demand therapy for 26 weeks, and then were treated with ALTUVIIIO prophylaxis for another 26 weeks.
This group also saw striking results. On average, people who switched from ALTUVIIIO on demand to ALTUVIIIO prophylaxis went from 21 bleeds to less than 1 bleed a year (mean ABR 0.7). That’s a 97% mean reduction in yearly bleeds.‡
And over the 26 weeks on ALTUVIIIO prophylaxis, 77% of people had 0 bleeds.‡
Both groups showed significant improvement in bleed protection with ALTUVIIIO prophylaxis.
‡Data based on treated bleeds.
3. FEEL CONFIDENT YOUR JOINTS ARE PROTECTED
The XTEND-1 study also examined target joint bleeds. When evaluating joint results at 52 weeks in the 128 people who participated in the XTEND-1 study, 72% of people had 0 joint bleeds‡ on prophylaxis after switching to ALTUVIIIO. 100% of target joints were resolved.
Target joints:
- Are 3 or more spontaneous bleeds in a major joint within a period of 6 consecutive months
- Were considered resolved if 2 or fewer bleeds occurred in the target joint within 12 months
4. THE FEWEST WEEKLY INFUSIONS AMONG FACTOR VIII PROPHYLAXIS TREATMENTS
While most people with hemophilia grow accustomed to infusing, fewer infusions are generally preferred. In studies, ALTUVIIIO clearly outlasted other Factor VIII replacement therapies, meaning ALTUVIIIO takes longer to be reduced by half in the body, and therefore lasts for a longer period.
So instead of needing up to 4 infusions a week with other treatments, patients on ALTUVIIIO infused only once per week.
ALTUVIIIO offers the fewest weekly infusions among Factor VIII prophylaxis treatments.
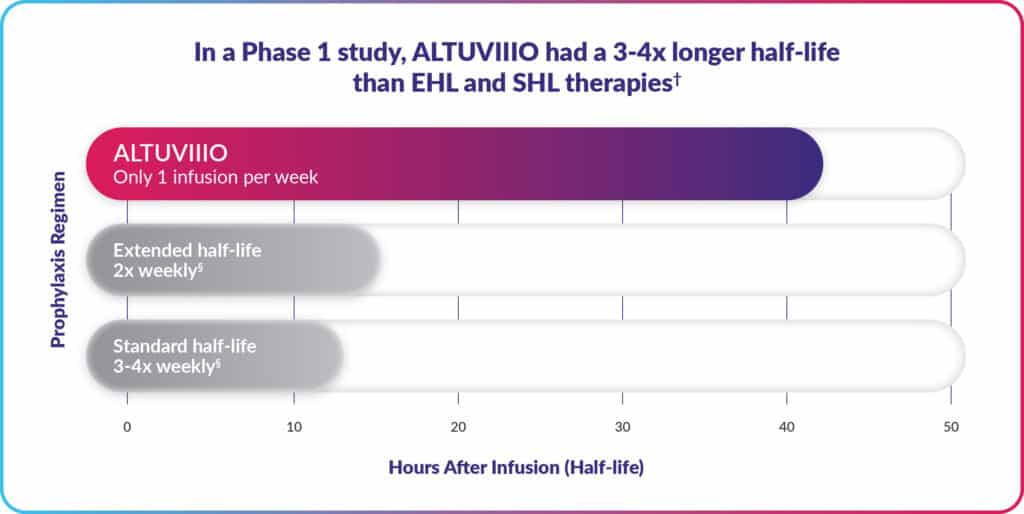
†This is information from a study in 13 previously treated adults with severe hemophilia A that had the goal of comparing how long ALTUVIIIO, Adynovate® [Antihemophilic Factor (Recombinant), PEGylated], and Advate® [Antihemophilic Factor (Recombinant)] stayed in the body after 1 dose. Half-life was 43 hours for ALTUVIIIO, 15 hours for Adynovate, and 11 hours for Advate.
§Doses and dosing intervals may be adjusted.
Adynovate and Advate are registered trademarks of Baxalta Incorporated, a Takeda company.
Now that you’ve learned about a few of the ways ALTUVIIIO protects you from bleeds, you may be considering a conversation with your doctor about your treatment plan. Our Doctor Discussion Guide can help. It offers a list of helpful questions to help you jump-start the conversation. You can also connect with your local Sanofi Community Relations and Education (CoRe) Manager, who can share additional resources and provide education.
INDICATION
ALTUVIIIO™ [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is an injectable medicine that is used to control and reduce the number of bleeding episodes in people with hemophilia A (congenital Factor VIII deficiency).
Your healthcare provider may give you ALTUVIIIO when you have surgery.
IMPORTANT SAFETY INFORMATION
What is the most important information I need to know about ALTUVIIIO?
Do not attempt to give yourself an injection unless you have been taught how by your healthcare provider or hemophilia center. You must carefully follow your healthcare provider’s instructions regarding the dose and schedule for injecting ALTUVIIIO so that your treatment will work best for you.
Who should not use ALTUVIIIO?
You should not use ALTUVIIIO if you have had an allergic reaction to it in the past.
What should I tell my healthcare provider before using ALTUVIIIO?
Tell your healthcare provider if you have had any medical problems, take any medications, including prescription and non-prescription medicines, supplements, or herbal medicines, are breastfeeding, or are pregnant or planning to become pregnant.
What are the possible side effects of ALTUVIIIO?
You can have an allergic reaction to ALTUVIIIO. Call your healthcare provider or emergency department right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash, or hives.
Your body can also make antibodies called “inhibitors” against ALTUVIIIO. This can stop ALTUVIIIO from working properly. Your healthcare provider may give you blood tests to check for inhibitors.
The common side effects of ALTUVIIIO are headache, joint pain, and back pain.
These are not the only possible side effects of ALTUVIIIO. Tell your healthcare provider about any side effect that bothers you or does not go away.
Please see full Prescribing Information.

© 2023 Genzyme Corporation. All rights reserved.
ALTUVIIIO and Sanofi are trademarks of Sanofi or an affiliate.
MAT-US-2209658-v1.0-05/2023

