This is a paid public announcement from CSL Behring and does not constitute an endorsement of products or services. When you click on the links in this blog entry, you will be directed to the CSL Behring website. LA Kelley Communications always advises you to be a savvy consumer when contacting any company; do not reveal identifying information against your will.
The current standard of care for moderate to severe hemophilia B, factor IX prophylactic therapy, does offer bleed protection. However, it requires lifelong, routine infusions to maintain protective factor levels, and those who regularly infuse factor IX replacement products can still experience breakthrough bleeds, leading to joint damage, pain, and reduced quality of life. There is a need for a treatment option that offers consistent bleed protection with a one-time infusion that lasts years instead of weeks.
After years of scientific research and clinical studies, that option is here—HEMGENIX® (etranacogene dezaparvovec-drlb), the first and only gene therapy for hemophilia B.*
Hemophilia B is an appropriate target for treatment with gene therapy
Hemophilia B is caused by a mutation of a single gene—the F9 gene. Approaches using gene therapy to treat inherited conditions stemming from a single genetic mutation, including hemophilia B, have predominantly focused on the delivery of a working, or functional, gene using a viral vector.
Hemophilia B is an appropriate target for treatment with gene therapy because it is caused by a mutation of a single gene, the F9 gene, which is small enough that it can be packaged into an adeno-associated viral (AAV) vector.
How HEMGENIX gene therapy for hemophilia B works
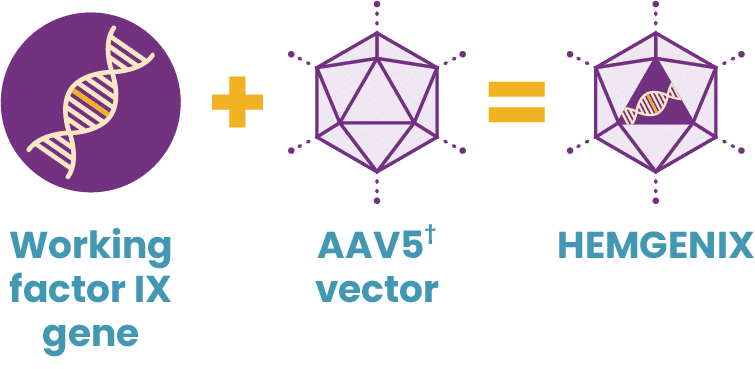
HEMGENIX uses a gene therapy approach called gene transfer. Gene transfer therapy for hemophilia B starts by developing a package of genetic instructions—the functional, or working, gene. Then AAV vectors are created, which will eventually enter targeted liver cells. The package of genetic instructions is loaded into an AAV vector shell, acting as a delivery truck. Through a single IV infusion, the delivery truck heads toward the liver with its package.
Once delivered into the liver cells, the package of instructions is unloaded, enabling the liver to start generating factor IX, with the goal of allowing a person to produce their own elevated and protective levels of factor IX. After delivering its package, the AAV vector shell is broken down and eliminated. However, the genetic instructions remain to continue producing factor IX.
Built on science you can trust
Gene therapy is built on decades of clinical research. The first patients received gene therapy in 1970, and there are more than 250 AAV-based clinical trials currently underway across a variety of conditions. So not only is gene therapy with HEMGENIX EMHa great fit for hemophilia B, it’s based on years of scientific research.
Interested in learning more about the science behind HEMGENIX, a one-time infusion that offers years of consistent bleed protection? Explore all that gene therapy might offer for people with hemophilia B today!
*HEMGENIX was studied in a clinical trial of 54 male adults with moderately severe or severe hemophilia B. All people in the trial were taking factor IX prophy to treat their hemophilia B and were observed for at least 6 months on prophy before receiving HEMGENIX.
†AAV5, adeno-associated viral vector serotype 5.
IMPORTANT SAFETY INFORMATION
What is HEMGENIX?
HEMGENIX®, etranacogene dezaparvovec-drlb, is a one-time gene therapy for the treatment of adults with hemophilia B who:
- Currently use Factor IX prophylaxis therapy, or
- Have current or historical life-threatening bleeding, or
- Have repeated, serious spontaneous bleeding episodes.
HEMGENIX is administered as a single intravenous infusion and can be administered only once.
What medical testing can I expect to be given before and after administration of HEMGENIX?
To determine your eligibility to receive HEMGENIX, you will be tested for Factor IX inhibitors. If this test result is positive, a retest will be performed 2 weeks later. If both tests are positive for Factor IX inhibitors, your doctor will not administer HEMGENIX to you. If, after administration of HEMGENIX, increased Factor IX activity is not achieved, or bleeding is not controlled, a post-dose test for Factor IX inhibitors will be performed.
HEMGENIX may lead to elevations of liver enzymes in the blood; therefore, ultrasound and other testing will be performed to check on liver health before HEMGENIX can be administered. Following administration of HEMGENIX, your doctor will monitor your liver enzyme levels weekly for at least 3 months. If you have preexisting risk factors for liver cancer, regular liver health testing will continue for 5 years post-administration. Treatment for elevated liver enzymes could include corticosteroids.
What were the most common side effects of HEMGENIX in clinical trials?
In clinical trials for HEMGENIX, the most common side effects reported in more than 5% of patients were liver enzyme elevations, headache, elevated levels of a certain blood enzyme, flu-like symptoms, infusion-related reactions, fatigue, nausea, and feeling unwell. These are not the only side effects possible. Tell your healthcare provider about any side effect you may experience.
What should I watch for during infusion with HEMGENIX?
Your doctor will monitor you for infusion-related reactions during administration of HEMGENIX, as well as for at least 3 hours after the infusion is complete. Symptoms may include chest tightness, headaches, abdominal pain, lightheadedness, flu-like symptoms, shivering, flushing, rash, and elevated blood pressure. If an infusion-related reaction occurs, the doctor may slow or stop the HEMGENIX infusion, resuming at a lower infusion rate once symptoms resolve.
What should I avoid after receiving HEMGENIX?
Small amounts of HEMGENIX may be present in your blood, semen, and other excreted/secreted materials, and it is not known how long this continues. You should not donate blood, organs, tissues, or cells for transplantation after receiving HEMGENIX.
Please see full prescribing information [LINK TO: https://labeling.cslbehring.com/PI/US/Hemgenix/EN/Hemgenix-Prescribing-Information.pdf] for HEMGENIX.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch [LINK TO: www.fda.gov/medwatch], or call 1-800-FDA-1088.
You can also report side effects to CSL Behring’s Pharmacovigilance Department at 1-866-915-6958.
HEMGENIX is manufactured by uniQure Inc. and distributed by CSL Behring LLC. HEMGENIX® is a registered trademark of CSL Behring LLC.
©2023 CSL Behring LLC 1020 First Avenue, PO Box 61501, King of Prussia, PA 19406-0901 USA
www.CSLBehring.com USA-HGX-0464-MAY23

